Tetranite is a novel bone adhesive that is transforming the standard of care for both patients and clinicians. In conjunction with our investigative partners, we are currently enrolling patients and conducting a range of studies in cities around the country (with more to come) run by our team of experienced clinicians, investigators and biotech engineers. If you are interested in participating in any of our current or future clinical trials, please review the information below. You may contact us or any of the local clinician offices listed below.
CURRENT STUDY: DENTAL IMPLANT STANDARD OF CARE STUDY
RevBio is currently conducting a Standard of Care Study which seeks to illustrate the effects on patients following Standard of Care treatments of extraction and delayed implant placements—when compared to the single stage procedure of TETRANITE Adhesive Dental Bone Scaffold with immediate implant stabilization. Participation in this study does not alter your clinician’s standard treatment recommendation.
NEED DENTAL IMPLANTS? Get pAid to PARTICIPATE in a clinical study designed to improve the Patient implant experience

BENEFITS FOR PATIENTS PARTICIPATING IN THE STUDY (See below)
The purpose of this Standard of Care Study is to gather information on patient experiences after tooth extraction and delayed implant placement. If you would like to participate, you will be helping both clinicians and RevBio, Inc. gather critical information regarding patient satisfaction with your treatment, i.e., tooth extraction with delayed dental implant placement. There is no comparable information in current scientific literature and this study is a first step to fill this gap in medical knowledge. Participating in this study will benefit the profession and more importantly, patient care.
STUDY PARTICIPATION PROCESS
| PATIENT INCENTIVE: | Patients will receive up to $500 upon study participation completion |
| STEP 1 | DISCUSS WITH YOUR DOCTOR THE SUITABILITY CRITERIA |
| STEP 2 | RECEIVE STANDARD OF CARE TREATMENT FOR TOOTH EXTRACTION PROCEDURES |
| STEP 3 | COMPLETE QUESTIONNAIRE #1 WITHIN 2-5 DAYS AFTER YOUR PROCEDURE |
| STEP 4 | COMPLETE QUESTIONNAIRE #2 WITHIN 7-10 DAYS AFTER YOUR PROCEDURE |
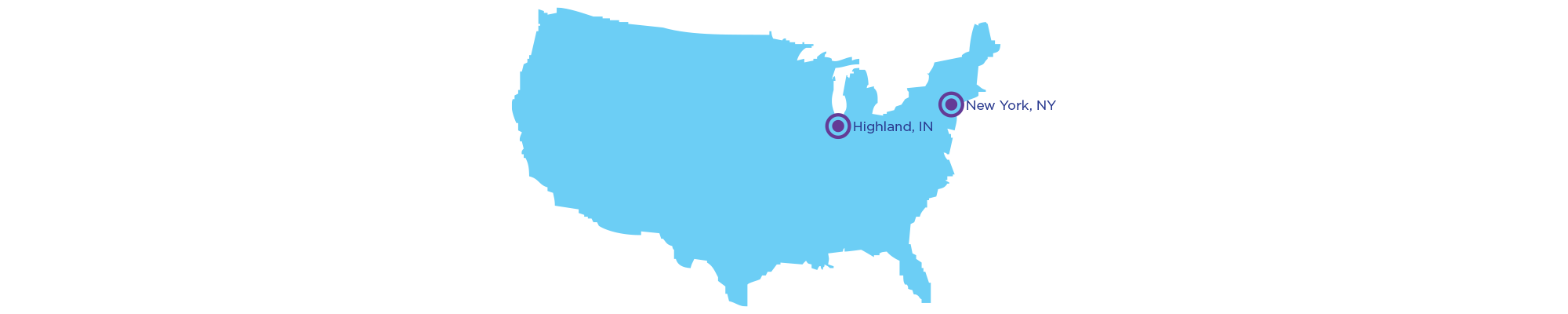
CURRENT CLINICAL STUDY SITES
The RevBio Dental Implant Standard of Care study is being conducted in New York by Dr. Alan Pollack, DDS and in Indiana by Dr. Eric Pulver, DDS. RevBio is the study sponsor for both locations.
PRINCIPAL INVESTIGATORS TO DATE

ALAN POLLACK, DDS
NEW YORK PERIODONTICS
225 E. 64th Street
New York, NY 10065
Office Number: (212) 838-0940
https://www.ppa-nyc.com

L. ERIC PULVER, DDS
PULVER ORAL SURGERY
2629 45th Street
Highland, IN 46322
Office Number: (219) 934-0404
https://www.pulveroralsurgery.com
If you think you may be a candidate for any of our clinical trials please contact us or the study location: